Dipole moment of a bond is a vector and physical quantity to calculate the percentage ionic character in a covalent bond. It is expressed as:
Dipole moment where, is dipole moment and $d$ is the bond length. It is usually expressed in terms of C.G.S. unit known as Debye (D) esu cm. In S.I. unit it is expressed in coulomb metre. Resultant dipole moment of two bond moments acting at an angle , is given by:
(molecule is non-polar)
If $\mu \neq 0$ molecule is polar. Dipole moment plays an important role in deciding the stability order of alkanes,i.e., a more stable alkane has less dipole moment. The dipole moment of a molecule can predict the geometrical and position isomers as well as orientations in benzene nucleus and polarity of molecule.
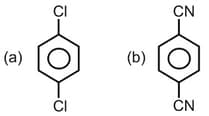
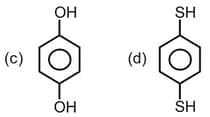
Which species is polar?
Important Questions on Chemical Bonding and Molecular Structure