Directions: Each of these questions contain an assertion followed by reason. Read them carefully and answer the question on the basis of the following options. You have to select the one that best describes the two statements.
Assertion: -Hydroxy benzene sulphonic acid, on heating to , changes to -Hydroxybenzene sulphonic acid.
Reason: Sulphonation of phenol is a reversible process.

Important Questions on Alcohols, Phenols and Ethers
Directions: Each of these questions contain an assertion followed by reason. Read them carefully and answer the question on the basis of the following options. You have to select the one that best describes the two statements.
Assertion: Phenol, on treatment with , gives tribromoderivatives, while with , it gives monobromo derivatives.
Reason: helps to increase the activity of the phenol ring.
Directions: Each of these questions contain an assertion followed by reason. Read them carefully and answer the question on the basis of the following options. You have to select the one that best describes the two statements.
Assertion: -Nitrophenol gives more electrophilic substituted compound than -methoxyphenol.
Reason: Methoxy group shows only effect.
Directions: Each of these questions contain an assertion followed by reason. Read them carefully and answer the question on the basis of the following options. You have to select the one that best describes the two statements.
Assertion: Salicylic acid may be prepared from sodium phenoxide by Kolbe-Schmidt reaction using carbon dioxide.
Reason: Kolbe-Schmidt reaction is a nucleophilic substitution reaction.
Directions: Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
Assertion: cleaves -diols but not - or higher diols.
Reason: Only -diols form cyclic periodate esters which subsequently undergo cleavage to form carbonyl compounds.
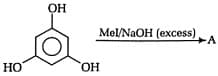
Final Product of given reaction: