Directions: Read the following paragraph and the figure given alongside to answer the following questions.
In radioactive decay, a nucleus decays into components. The atomic mass of the nucleus must be equal to the sum of the atomic masses of the components. Also, the atomic number of the nucleus must be equal to the sum of the atomic numbers of the components. A component of decay may be a beta particle that has an atomic number - and atomic mass (zero). The particle is denoted as Ceo), where the subscript refers to the atomic number and the superscript refers to the atomic mass. Another example of a decay particle is an alpha particle which is denoted as 2He4 . The graph given here represents a nucleus (a) undergoing many stages of decay.
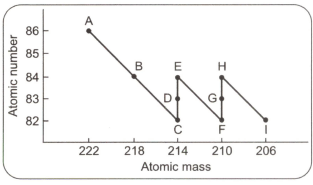
The scheme indicates that a stable nucleus may be formed which has a mass of:

Important Questions on Learning
Fill in the blanks.
The committee ____________ of all the changes in the report.
Teachers' code of conduct is ____.
In the context of Indian society, teaching is more than a challenge because:
An important part of the teaching learning process is the curriculum. This curriculum should be prepared keeping in view the _____.
In philosophy, different philosophies are studied _____.

