Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
Benzaldehyde undergoes disproportionation reaction in basic medium.
Aldehydes which do not have -hydrogen undergo Cannizzaro reaction (i.e., disproportionation reaction).
Benzaldehyde undergoes disproportionation reaction in basic medium.

Important Questions on Aldehydes, Ketones and Carboxylic Acids
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
The addition of ammonia derivative to a carbonyl compound is carried out in weakly acidic medium.
In weakly acidic medium attacking nucleophile is also protonated.
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
Acetic acid does not give haloform reaction.
Acetic acid has no hydrogen.
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
Formic acid reduces mercuric chloride.
Formic acid has reducing aldehydic group.
Each of these questions contains an Assertion followed by a reason. Read them carefully and answer the question on the basis of the following options. You have to select the one that best describes the two statements.
Carboxylic acids have a carbonyl group, but they do not give the test of the carbonyl group.
Due to resonance, the double bond character of carbonyl group is greatly reduced.
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
The order of base catalysed hydrolysis of ester is
(1)
(2) 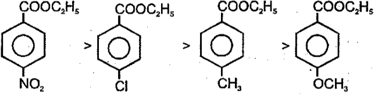
The reaction is sterically as well as electronically controlled reaction.
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
Acid catalysed hydrolysis of ester is reversible while base catalysed hydrolysis is irreversible.
In acid catalysèd ester hydrolysis carboxylic acid is formed on which nucleophilic attack of alcohol is possible but in base catalysed ester hydrolysis carboxylate anion is formed on which nucleophilic attack is not possible.
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
Acetate ion is more basic than the methoxide ion.
The methoxide ion is resonance stabilized.
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
Hydroxybenzoic acid has a lower boiling point than hydroxybenzoic acid.
Hydroxybenzoic acid has intermolecular hydrogen bonding.
