Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
Chloral hydrate is stable.
It is stable due to its high molecular weight.
Chloral hydrate is stable.

Important Questions on Aldehydes, Ketones and Carboxylic Acids
Each of these questions contains an Assertion followed by a reason. Read them carefully and answer the question on the basis of the following options. You have to select the one that best describes the two statements.
Cyclopropanone undergoes addition with more easily in comparison to acetone
Cyclopropanone contain strained ring and also has less steric crowding.
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
converts Acetone into propane but cannot.
converts acetone into pinacol.
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
Benzaldehyde undergoes disproportionation reaction in basic medium.
Aldehydes which do not have -hydrogen undergo Cannizzaro reaction (i.e., disproportionation reaction).
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
The addition of ammonia derivative to a carbonyl compound is carried out in weakly acidic medium.
In weakly acidic medium attacking nucleophile is also protonated.
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
Acetic acid does not give haloform reaction.
Acetic acid has no hydrogen.
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
Formic acid reduces mercuric chloride.
Formic acid has reducing aldehydic group.
Each of these questions contains an Assertion followed by a reason. Read them carefully and answer the question on the basis of the following options. You have to select the one that best describes the two statements.
Carboxylic acids have a carbonyl group, but they do not give the test of the carbonyl group.
Due to resonance, the double bond character of carbonyl group is greatly reduced.
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
The order of base catalysed hydrolysis of ester is
(1)
(2) 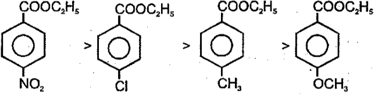
The reaction is sterically as well as electronically controlled reaction.
