Does the drawing represent a solid or a liquid? Give two reasons for your answer.
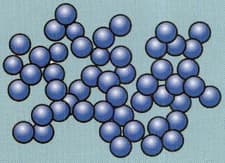

Important Questions on Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.

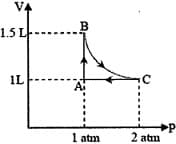
The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:

[Heat of fusion of ice ; Specific heat of water ]
(R = 8.314 J/mol K) (ln7.5 = 2.01)
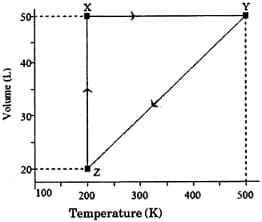
The pressure of the gas (in atm) at and respectively, are
The combination of plots which does not represent isothermal expansion of an ideal gas is




Which of the following statements are true?
I. On heating the kinetic energy of particles in solids does not change because they have a fixed position.
II. Sublimation is the change of gaseous state directly to solid state without going through liquid state and vice versa.
III. The movement of particles from an area of higher concentration to lower concentration is called diffusion.
IV. The rate of evaporation is not affected by increasing the temperature.
The three processes in a thermodynamic cycle shown in the figure are : Process is isothermal; Process is isochoric (volume remains constant); Process is adiabatic.
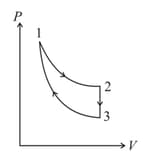
The total work done by the ideal gas in this cycle is, The internal energy decreases by, in the isochoric process. The work done by the gas in the adiabatic process is, . The heat added to the system in the isothermal process is

The correct option(s) is (are)
(Latent heat of ice is and )

