MEDIUM
Earn 100
Does the presence of two chiral carbon atoms always make the molecule optically active? Explain giving an example?
Important Questions on Basic Principles of Organic Chemistry
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
EASY
EASY
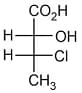 is:
is:EASY
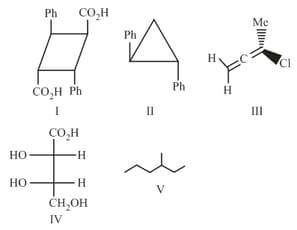
the compounds which can exhibit optical activity are :
MEDIUM
Which of the following alkenes can generate optically active compounds upon hydrogenation?
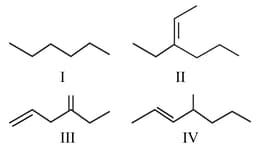
MEDIUM
EASY
MEDIUM
EASY
HARD
The Fischer projection of -erythrose is shown below.
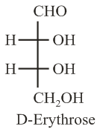
-Erythrose and its isomers are listed as and in Column I. Choose the correct relationship of and with -erythrose from Column II.
| Column - I | Column -II | ||
| P. | 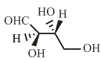 |
1. | Diastereomer |
| Q. | 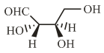 |
2. | Identical |
| R. | 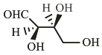 |
3. | Enantiomer |
| S. | 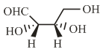 |
EASY
EASY
EASY
EASY
MEDIUM
EASY
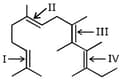
Geometrical isomerism is not possible at the site(s):

