EASY
Earn 100
Draw Sawhorse projections for the eclipsed and staggered conformations of ethane. Which of these conformations is more stable and why?
Important Questions on Hydrocarbons
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
Which hydrogen in compound is easily replaceable during bromination reaction in presence of light ?
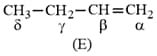
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
Arrange the following conformational isomers of n-butane in order of their increasing potential energy:
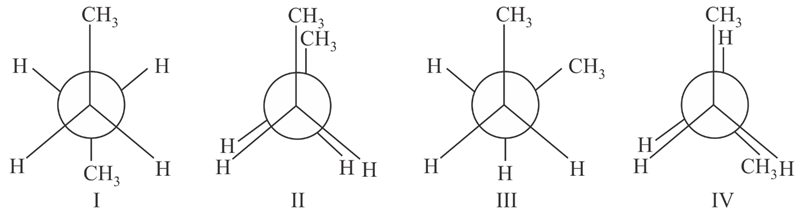
MEDIUM
MEDIUM
MEDIUM
EASY
EASY

