MEDIUM
10th ICSE
IMPORTANT
Earn 100
Draw a labelled diagram of the apparatus you would use to determine the specific latent heat of vaporisation of steam by the method of mixture. State two precautions you would take while performing the experiment with the apparatus.

Important Questions on Calorimetry
MEDIUM
10th ICSE
IMPORTANT
MEDIUM
10th ICSE
IMPORTANT
MEDIUM
10th ICSE
IMPORTANT
MEDIUM
10th ICSE
IMPORTANT
MEDIUM
10th ICSE
IMPORTANT
The graph represents a cooling curve for a substance being cooled from higher temperature to a lower temperature.What is the boiling point of the substance?
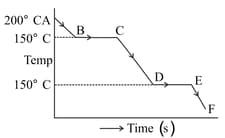
EASY
10th ICSE
IMPORTANT
The graph represents a cooling curve for a substance being cooled from higher temperature to a lower temperature.What happens in the region DE. Why is the region DE shorter than the region BC?
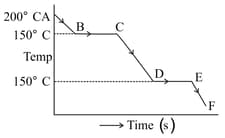
MEDIUM
10th ICSE
IMPORTANT
MEDIUM
10th ICSE
IMPORTANT
