MEDIUM
12th Maharashtra Board
IMPORTANT
Earn 100
Draw a p-V diagram and explain the concept of positive and negative work. Give one example each.

Important Questions on Thermodynamics
EASY
12th Maharashtra Board
IMPORTANT
HARD
12th Maharashtra Board
IMPORTANT
A gas contained in a cylinder fitted with a frictionless piston expands against a constant external pressure of from a volume of
to a volume of . In doing so it absorbs of thermal energy from its surroundings. Determine the change in internal energy of system.
EASY
12th Maharashtra Board
IMPORTANT
MEDIUM
12th Maharashtra Board
IMPORTANT
HARD
12th Maharashtra Board
IMPORTANT
MEDIUM
12th Maharashtra Board
IMPORTANT
An ideal gas is taken through an isothermal process. If it does of work on its environment, how much heat is added to it?
HARD
12th Maharashtra Board
IMPORTANT
HARD
12th Maharashtra Board
IMPORTANT
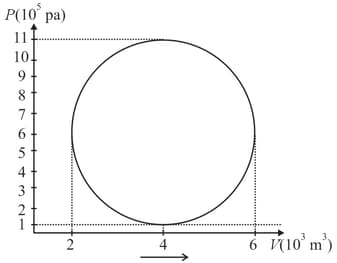
A hypothetical thermodynamic cycle is shown in the figure. Calculate the work done in 25 cycles.