HARD
Earn 100
Draw a well labelled diagram for testing the conductivity of salt solution.
Important Questions on Electrochemistry
EASY
EASY
Addition of excess of to an aqueous solution of mole of gives moles of . The conductivity of this solution corresponds to:
EASY
The variation of molar conductively with concentration of an electrolyte (X) in aqueous solution is shown in the given figure.
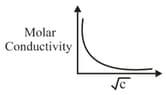
The electrolyte X is :
MEDIUM
MEDIUM
HARD
MEDIUM
EASY
MEDIUM
HARD
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
EASY
MEDIUM
EASY
: Conductivity always increases with decreases in the concentration of electrolyte.
: Molar conductivity always increases with decreases in the concentration of electrolyte.
The correct option among the following
EASY
EASY
The group of compounds which dissociate partially in aqueous solutions is

