HARD
Upper Secondary-IGCSE
IMPORTANT
Earn 100
Draw an ionic diagram similar to the one above for the structure of magnesium chloride.

Important Questions on Elements and Compounds
HARD
Upper Secondary-IGCSE
IMPORTANT
Draw dot and cross structural diagram to represent the bonding in the following simple molecular compounds. In the dot and cross diagram, show only the outer shells of the atoms involved.
| Molecule | Dot and Cross diagram | Structure |
| Ammonia | ||
| Water | ||
| Hydrogen chloride | ||
| Ethane | ||
| Ethene | ||
| Ethanol | ||
| Ethanoic acid |
MEDIUM
Upper Secondary-IGCSE
IMPORTANT
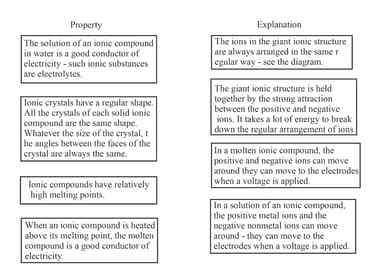
The boxes below contain properties of ionic compounds and their explanations.Draw lines to link each pair.
HARD
Upper Secondary-IGCSE
IMPORTANT
| Observation | Explanation |
| Diamond and silica are both very hard substances _____ . | _____ because all the atoms in the structure are joined by strong covalent bonds. |
| Diamond does not conduct electricity _____ . | _____ because |
| Graphite is _____ . | _____ because the layers in the structure are only held together by weak forces |
| _____ because there are some free electrons that are able to move between the layers to carry the current. |
HARD
Upper Secondary-IGCSE
IMPORTANT
HARD
Upper Secondary-IGCSE
IMPORTANT
HARD
Upper Secondary-IGCSE
IMPORTANT
HARD
Upper Secondary-IGCSE
IMPORTANT
HARD
Upper Secondary-IGCSE
IMPORTANT
