MEDIUM
8th ICSE
IMPORTANT
Earn 100
Draw the diagram representing the atomic structure of the following:
Carbon atom (Atomic number 6; Mass No. 12).

Important Questions on Atomic Structure
MEDIUM
8th ICSE
IMPORTANT
MEDIUM
8th ICSE
IMPORTANT
HARD
8th ICSE
IMPORTANT
HARD
8th ICSE
IMPORTANT
HARD
8th ICSE
IMPORTANT
The electronic configuration of two elements X and Y are given below
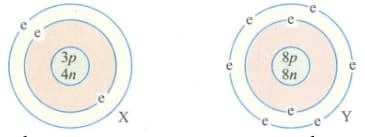
where p = proton, n = neutron, e = electron. When these two elements combine together to form a compound, give the mass (in grams) of one mole of this compound.
