HARD
JEE Main/Advance
IMPORTANT
Earn 100
Dry air was successively passed through a solution of solute in water and then through pure water. The loss in weight of solution was and that of pure water was What is mol. wt of solute?
50% studentsanswered this correctly

Important Questions on Solutions
MEDIUM
JEE Main/Advance
IMPORTANT
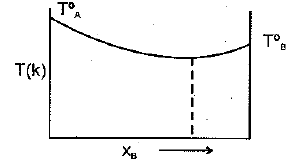
The diagram given below represents boiling point composition diagram of solution of components and which is/are incorrect among the following?
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
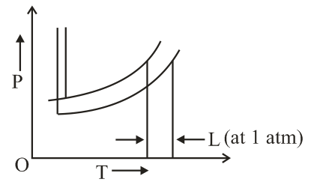
The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute, dashed line) are recorded below: The quantity indicated by in the figure is:
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
Three different ideal solutions (I, II, III) each containing total moles of in different composition are taken as shown in figure and pressure over the solutions is gradually reduced.
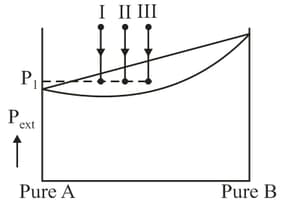
Initially external pressure is same for all three solutions. At a particular external pressure , ll solution is found to have
Then select correct statement:
EASY
JEE Main/Advance
IMPORTANT
