EASY
NEET
IMPORTANT
Earn 100
Due to capillary action, a liquid will rise in a tube, if the angle of contact is
(a)acute
(b)obtuse
(c)
(d)
50% studentsanswered this correctly

Important Questions on Viscosity and Surface Tension
EASY
NEET
IMPORTANT
A few droplets merge with each other to form a large droplet. In this process,
EASY
NEET
IMPORTANT
Two capillary tubes of the same diameter are put vertically, one each in two liquids whose relative densities are and and surface tensions are and , respectively. Ratio of heights of liquids in the two tubes is,
EASY
NEET
IMPORTANT
The property utilised in the manufacture of lead shots is
EASY
NEET
IMPORTANT
Surface tension of a liquid is . If its thin film is made in a ring of area , then its surface energy will be,
EASY
NEET
IMPORTANT
If one end of capillary tube is dipped into water, then water rises up to . If the surface tension of water is , then the diameter of capillary tube will be,
EASY
NEET
IMPORTANT
If the surface tension of a liquid is and its surface area is increased by , then the surface energy of that surface will be increased by,
EASY
NEET
IMPORTANT
Two soap bubbles of radii equal to are touching each other over a common surface (shown in figure). Its radius will be,
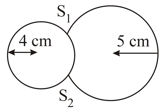
MEDIUM
NEET
IMPORTANT
The radius of a soap bubble is . The surface tension of soap solution is . Keeping the temperature constant, if the radius of the soap bubble is doubled, then the energy necessary for this will be,
