HARD
NEET
IMPORTANT
Earn 100
During the course of a chemical reaction, the rate of a reaction
(a)Remains constant throughout
(b)Increases as the reaction proceeds
(c)Decreases as the reaction proceeds
(d)First increases followed by a decrease
50% studentsanswered this correctly

Important Questions on Chemical Kinetics
EASY
NEET
IMPORTANT
HARD
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
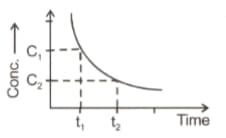
EASY
NEET
IMPORTANT
Which of the following is correct?
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
The rate law of a reaction between the substances and is given by, rate . On doubling the concentration of $A$ and making the volume of half the ratio of new rate to the earlier rate of reaction will be
