During which of the following processes, does entropy decrease ?
(A) Freezing of water to ice at
(B) Freezing of water to ice at
(C)
(D) Adsorption of and lead surface
(E) Dissolution of in water
(B) Freezing of water to ice at
(C)
(D) Adsorption of and lead surface
(E) Dissolution of in water

Important Questions on Thermodynamics
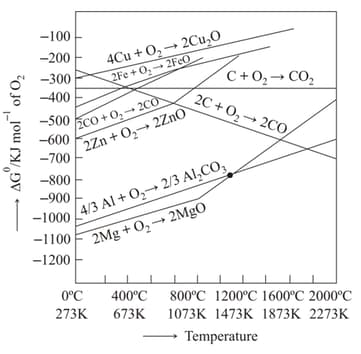
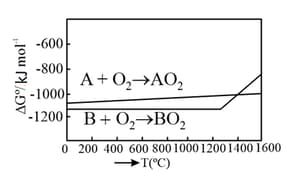
The point of intersection and sudden increase in the slope, in the diagram given below, respectively, indicates:
The standard enthalpies of formation of and are and respectively.
For the reaction
the standard reaction enthalpy ________
(Round off to the Nearest Integer).
At of iron reacts with to form . The evolved hydrogen gas expands against a constant pressure of . The work done by the gas during this expansion is -_______ .
(Round off to the Nearest Integer)
[Given : . Assume, hydrogen is an ideal gas]
[Atomic mass off Fe is ]
For the reaction at ,
If we start the reaction in a closed container at with millimoles of , the amount of is the equilibrium mixture is ________ millimoles. (Round off to the Nearest Integer).
(Given,
(Specific heat of water liquid and water vapour are and ; heat of liquid fusion and vaporization of water are and , respectively). ( )
