Electrolysis of water is a decomposition reaction. The mole ratio of hydrogen and oxygen gases liberated during electrolysis of water is:

Important Questions on Chemical Reactions And Equations
Among the following, the number of underlined elements having oxidation state are:
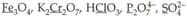
Nandita mixed two solutions and . She recorded the following observations and conclusions in her notebook.
A yellow precipitate is formed.
It is a double displacement reaction.
The solutions and respectively are:
Which of the following statements is wrong relating to the above equation?
Which of the following are exothermic processes?
Reaction of water with quick lime.
Dilution of an acid.
Evaporation of water.
Sublimation of camphor.
Four students and noted the initial colour of the solution kept in beakers and . After inserting zinc rod in each solution and leaving them undisturbed for two hours, the colour of each solution was again noted in the given table.
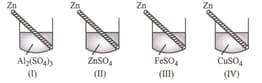
| Student | Colour | ||||
|
Initial Final |
Light green Colourless |
Colourless Colourless |
Light green Dark green |
Blue Colourless |
|
|
Initial Final |
Colourless Colourless |
Light yellow Colourless |
Light green Light green |
Blue Colourless |
|
|
Initial Final |
Colourless Light blue |
Colourless Colourless |
Light green Colourless |
Blue Light blue |
|
|
Initial Final |
Colourless Colourless |
Colourless Colourless |
Light green Colourless |
Blue Colourless |
Which student observed the colour change in all the four beakers correctly?
