Electronic displacement in a covalent bond is represented by the following notation.
Is the displacement of electrons in a covalent bond temporary or permanent.

Important Questions on Basic Principles of Organic Chemistry
A covalent bond in tert-butylbromide breaks in a suitable polar solvent to give ions. Name the anion produced by this breaking of a covalent bond.
A covalent bond in tert-butylbromide breaks in suitable polar solvent to give ions. Indicate the type of bond breaking in this case.
A covalent bond in tert-butylbromide breaks in a suitable polar solvent to give ions. Comment on the geometry of the cation formed by such bond cleavage.
Which of the following statements are true with respect to electronic displacement in a covalent bond?
a. The inductive effect operates through bond
b. The resonance effect operates through bond
c. The inductive effect operates through bond
d. Resonance effect operates through bond
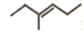 is _____.
is _____.