MEDIUM
AS and A Level
IMPORTANT
Earn 100
Element having electronic configuration: forms an ion of type .
Write the symbol for the sub-shell that begins to fill after the and are completely full.

Important Questions on Electrons in Atoms
MEDIUM
AS and A Level
IMPORTANT
Define the following: Ionization energy.
EASY
AS and A Level
IMPORTANT
Define the following: Ionization energy.
MEDIUM
AS and A Level
IMPORTANT
Give the equation representing the Ionization energy of Magnesium.
MEDIUM
AS and A Level
IMPORTANT
Give the equation representing the ionization energy of magnesium.
MEDIUM
AS and A Level
IMPORTANT
State which ionization energies are represented by the equations below.
MEDIUM
AS and A Level
IMPORTANT
State which ionization energies are represented by the equations blow.
MEDIUM
AS and A Level
IMPORTANT
The graph shows a sketch of (ionization energy) against number of electrons removed for magnesium.
Use this graph to answer the following questions.
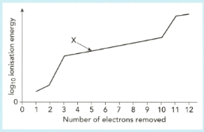
Explain why the first two electrons are relatively easy to remove.
MEDIUM
AS and A Level
IMPORTANT
The graph shows a sketch of (ionization energy) against number of electrons removed for magnesium.
Use this graph to answer the following questions.
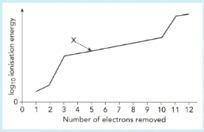
Explain why there is a sharp rise in ionisation energy when the third electron is removed.
