Elements calcium, strontium and barium were put in one group on the basis of their similar properties. What is the usual name of this group?
Important Questions on Periodic classification of elements
Element forms a chloride with formula . Element would be most likely in the same group of periodic table as:
Four elements W, X, Y, and Z have atomic numbers 12,18, 4, and 16:
Which element represents an inert gas?
Four elements W, X, Y, and Z have atomic numbers 12,18, 4, and 16:
Which element will be larger in size W or Z?
Give scientific reasons:
Elements belonging to the same group have the same valency.
What is the basis of the classification of elements of the modern periodic table?
Four elements W, X, Y, and Z have atomic numbers 12,18, 4, and 16:
Which element belongs to the second period?
The position of elements A, B, and C in the Periodic Table are shown below.
| Group 16 | Group 17 |
| - | - |
| - | - |
| - | A |
| B | C |
Will C be larger or smaller in size than B.
The following table shows the position of six elements A, B, C, D, E, and F.in the modern periodic table:
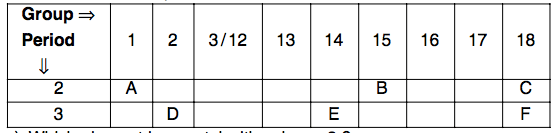
Which element is a metal with valency 2?
The following table shows the position of six elements A, B, C, D, E, and F.in the modern periodic table:
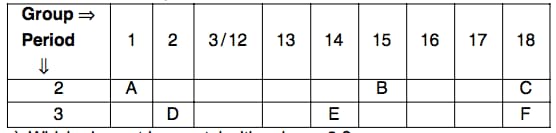
Which element is a non-metal with valency 3?
The position of elements A, B, and C in the Periodic Table are shown below.
| Group 16 | Group 17 |
| - | - |
| - | - |
| - | A |
| B | C |
State whether C is more reactive or less reactive than A.
The position of elements A, B, and C in the Periodic Table are shown below.
| Group 16 | Group 17 |
| - | - |
| - | - |
| - | A |
| B | C |
State whether A is a metal or non-metal.
The following table shows the position of six elements A, B, C, D, E, and F.in the modern periodic table:
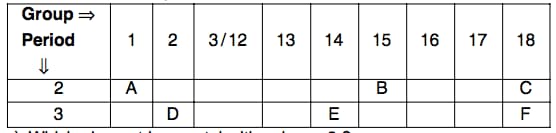
Out of D and E which one has a bigger atomic radius and why?

