MEDIUM
Earn 100
Elements of Group are called .
50% studentsanswered this correctly
Important Questions on The s-Block Elements
EASY
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
HARD
HARD
EASY
MEDIUM
MEDIUM
(A)
(B)
(C)
(D)
MEDIUM
MEDIUM
| List-I (Salt) | List-II (Flame colour wavelength) | ||
| (a) | (i) | ||
| (b) | (ii) | ||
| (c) | (iii) | ||
| (d) | (iv) | ||
Choose the correct answer from the options given below:
MEDIUM
EASY
MEDIUM
EASY
EASY
EASY
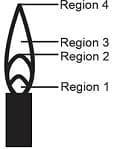
EASY

