EASY
NEET
IMPORTANT
Earn 100
Energy of a hydrogen atom with principal quantum number is given by . The energy of a photon ejected when the electron jumps from state to state of hydrogen is approximately:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Atomic Physics
EASY
NEET
IMPORTANT
HARD
NEET
IMPORTANT
Ionization potential of hydrogen atom is . Hydrogen atoms in the ground state are excited by monochromatic radiation of photon energy . According to Bohr's theory, the spectral lines emitted by hydrogen will be
MEDIUM
NEET
IMPORTANT
The diagram shows the energy levels for an electron in a certain atom. Which transition shown represents the emission of a photon with the most energy?
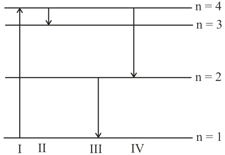
EASY
NEET
IMPORTANT
HARD
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
Energy levels and of a certain atom corresponding to increasing values of energy, i.e., . If and are the wavelengths of radiations corresponding to the transitions to to and to respectively, which of the following statements is correct?
