MEDIUM
11th CBSE
IMPORTANT
Earn 100
Enthalpy change of is negative. If enthalpy of combustion of and are and respectively, then which relation is correct?
(a)
(b)
(c)
(d)
14.29% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
Consider the reaction
If is formed instead of in the above reaction, the value will be (given, of sublimation for is )
MEDIUM
11th CBSE
IMPORTANT
Consider the following processes :
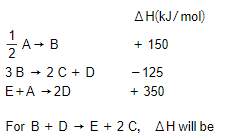
MEDIUM
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
Given-
Based on the above thermochemical equations, the value of
at for the reaction + will be
MEDIUM
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
