MEDIUM
Earn 100
Equal volumes of 0.002 M solutions of sodium iodate and cupric chlorate are mixed together. Will it lead precipitation of copper iodate? (For cupric iodate Ksp = 7.4 × 10-8). If the value of Ionic product of CU(SO3)2 in the reaction mixture is Yx 10-9. Find Y.
50% studentsanswered this correctly
Important Questions on Ionic Equilibrium
MEDIUM
MEDIUM
HARD
MEDIUM
EASY
MEDIUM
HARD
MEDIUM
HARD
HARD
MEDIUM
MEDIUM
[Solubility product for ]
MEDIUM
Which of the following choices is correct for a mixture of and
MEDIUM
MEDIUM
The stoichiometry and solubility product of a salt with the solubility curve given below is, respectively:
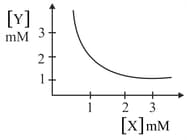
EASY
EASY
MEDIUM
HARD
EASY

