HARD
Earn 100
Ernest Rutherford devised an experiment in which alpha particles were directed at a thin gold foil. The results of this experiment showed that every atom has a nucleus, and the plum pudding model of the atom had to be discarded.
Draw a diagram to show how an alpha particle could be scattered backward by a gold atom, towards the source from which it came. Explain why most alpha particles passed straight through the gold foil.
Important Questions on Atoms
EASY
EASY
EASY
EASY
HARD
MEDIUM
EASY
EASY
Scattering angle
Number of scattered -particles detected
(Plots are schematic and not to scale)
EASY
MEDIUM
MEDIUM
MEDIUM
(Planck's constant electron mass )
EASY
MEDIUM
MEDIUM
HARD
EASY
A beam of fast moving alpha particles were directed towards a thin film of Gold. The parts and of the transmitted and reflected beams corresponding to the incident parts and of the beam are shown in the adjoing diagram. The number of alpha particles in
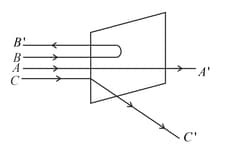
MEDIUM
HARD
EASY

