HARD
Upper Secondary: IGCSE
IMPORTANT
Earn 100
Ernest Rutherford devised an experiment in which alpha particles were directed at a thin gold foil. The results of this experiment showed that every atom has a nucleus, and the plum pudding model of the atom had to be discarded. Draw a diagram to show how an alpha particle could be scattered backward by a gold atom, towards the source from which it came.

Important Questions on The Nuclear Atom
HARD
Upper Secondary: IGCSE
IMPORTANT
Ernest Rutherford devised an experiment in which alpha particles were directed at a thin gold foil. The results of this experiment showed that every atom has a nucleus, and the plum pudding model of the atom had to be discarded. Explain why most alpha particles passed straight through the gold foil.
MEDIUM
Upper Secondary: IGCSE
IMPORTANT
The diagram represents a neutral lithium atom. All the particles in the atom are shown on the diagram. Use the diagram to help you answer the following question. How many electrons does this atom have?
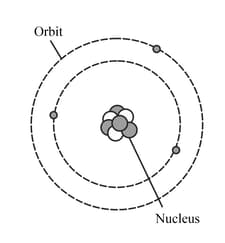
HARD
Upper Secondary: IGCSE
IMPORTANT
The diagram represents a neutral lithium atom. All the particles in the atom are shown on the diagram. Use the diagram to help you answer the following question. What is the value of the proton number of this atom?
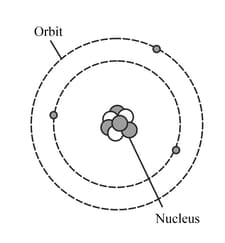
HARD
Upper Secondary: IGCSE
IMPORTANT
The diagram represents a neutral lithium atom. All the particles in the atom are shown on the diagram. Use the diagram to help you answer the following question. How many neutrons does the atom have?
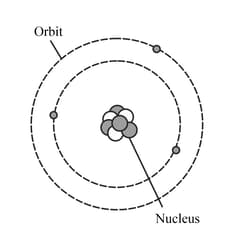
HARD
Upper Secondary: IGCSE
IMPORTANT
The diagram represents a neutral lithium atom. All the particles in the atom are shown on the diagram. Use the diagram to help you answer the following question. What is the value of the nucleon number of this atom?
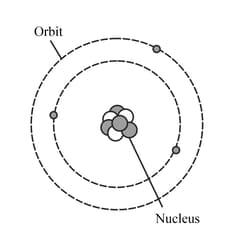
HARD
Upper Secondary: IGCSE
IMPORTANT
Copy the diagram below, replacing the boxes with appropriate numbers, to represent this atom of lithium in nuclide notation.
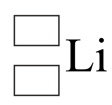 .
.HARD
Upper Secondary: IGCSE
IMPORTANT
The charges on the particles in an atom may be represented by or or ,
The masses of the particles in an atom may be represented by or or .
Using these choices, copy and complete the table.
| Particle | Charge | Mass |
| Electron | ||
| Neutron | ||
| Proton |
MEDIUM
Upper Secondary: IGCSE
IMPORTANT
How many of these particles are there in a neutral atom of
Electrons.
