Esterification of a carboxylic acid with an alcohol in the presence of mineral acid as catalyst is a reversible reaction.
If one mole of ethanoic acid and one mole of ethanol are allowed to reach equilibrium at , how many moles of ethyl ethanoate and ethanoic acid are present at equilibrium? (Assume )
Important Questions on Equilibrium
Which of the following statements is incorrect?
Using the Le Chatelier's principle, predict which one of the following will not disturb the equilibrium?
at is given below.
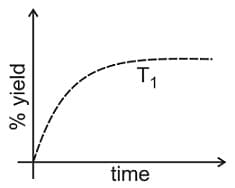
If this reaction is conducted at with , the % yield of ammonia as a function of time is represented by:
Assertion: The of water increases with increase in temperature.
Reason: The dissociation of water into and is an exothermic reaction.
The equilibrium shifts in forward direction:
In a reaction Le Chatelier's principle asserts that an equilibrium between and producing and can be shifted towards and by
(i) increasing the concentration of or
(ii)increasing the concentration of or
(iii) decreasing the concentration of or
The total pressure when both the solids dissociate simultaneously is:
Consider the following reaction:
For each of the following cases , the direction in which the equilibrium shifts is:
(a) Temperature is decreased.
(b) Pressure is increased by adding at constant T.
The volume of a closed reaction vessel in which the following equilibrium reaction occurs is halved.
As a result,
Following lists contain reactions and their corresponding equilibrium constants at different temperatures:
| List - I (Reaction) | List - II |
If are the standard enthalpies for the reactions respectively, then:
The equilibrium constant cannot be disturbed by

