EASY
Earn 100
Ethoxide ion is a stronger base than hydroxide ion.
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Alcohols, Phenols and Ethers
EASY
MEDIUM
Which is the most suitable reagent for the following transformation?
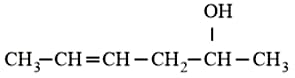
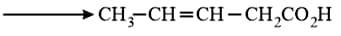
HARD
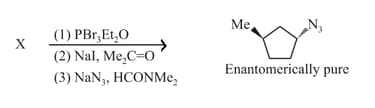
EASY
MEDIUM
MEDIUM
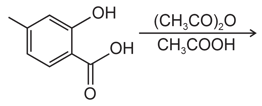
EASY
Among the following, which one cannot be formed as a product under any conditions?
MEDIUM
MEDIUM
and respectively, are
EASY
EASY
MEDIUM
Write mechanism of the following reaction :
MEDIUM
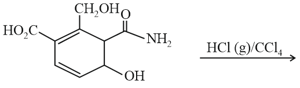
MEDIUM
MEDIUM
HARD
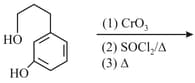
MEDIUM
The major product of the following reaction is
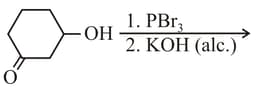
MEDIUM
HARD
Ethanol to Propan-2-ol
HARD

