EASY
MHT-CET
IMPORTANT
Earn 100
Experimentally determined molecular weight of acetic acid in benzene, comes out to be twice of its original molecular weight. This is because of _______.
(a)resonance structure
(b)intramolecular hydrogen bonding
(c)formation of dimer due to intermolecular bonding
(d)presence of carbonyl group
50% studentsanswered this correctly

Important Questions on Model Question Papers
EASY
MHT-CET
IMPORTANT
Reduction of
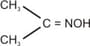 by and yields _______.
by and yields _______.EASY
MHT-CET
IMPORTANT
The magnetic moment of a complex ion is . The complex ion is ______.
EASY
MHT-CET
IMPORTANT
Chlorine dissolves in water in absence of sunlight and at ordinary temperatures to form compounds and . The compound decomposes on exposure to sunlight to give compound and nascent oxygen. The compounds and are ______, respectively.
EASY
MHT-CET
IMPORTANT
In , carbon atoms form ______.
EASY
MHT-CET
IMPORTANT
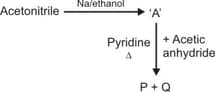
In the above reaction, and are ______ respectively.
EASY
MHT-CET
IMPORTANT
Acetone and benzene can be separated from their mixture by ______.
EASY
MHT-CET
IMPORTANT
For making tyres, _______ is added to natural rubber during vulcanization.
MEDIUM
MHT-CET
IMPORTANT
If
,
the possible heat of the formation of methane will be ______.
