MEDIUM
JEE Main
IMPORTANT
Earn 100
Explain:
Why do and exhibit similar properties?

Important Questions on d- and f-Block Elements
MEDIUM
JEE Main
IMPORTANT
The outer electronic configuration of two members of the lanthanide series are as follows:
and .
What are their atomic numbers? Predict the oxidation states exhibited by these elements in their compounds.
EASY
JEE Main
IMPORTANT
Chemistry of all the lanthanoids is quite similar. Explain by giving a reason.
MEDIUM
JEE Main
IMPORTANT
Which of the following graphs shows correct trends in the size of ions of lanthanoids?
MEDIUM
JEE Main
IMPORTANT
Select the correct match:
MEDIUM
JEE Main
IMPORTANT
Gadolinium has electronic configuration outside the core. Find spin magnetic moment of .
MEDIUM
JEE Main
IMPORTANT
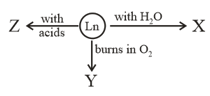
The and , respectively are
MEDIUM
JEE Main
IMPORTANT
All actinoids have high density except
MEDIUM
JEE Main
IMPORTANT
Choose the correct statements:
The size of the lanthanoid ions decreases as the atomic number of increases.
Electronic spectra of lanthanoid shows very broad bands.
As with transition metals, coordination number six is very common in lanthanoid complexes.
