Explain Thin layer chromatography (TLC) and Partition chromatography.
Important Questions on Organic Chemistry- Some Basic Principles and Techniques
A homogeneous mixture of compounds , and is spotted on a thin layer chromatography () plate. The plate is made of acidic silica. The spotted plate is placed in a closed jar containing diethyl ether as a mobile phase (eluent). As the eluent rises up the plate, the compounds of the mixture separate and travel to different distances. The developed plate is shown below.
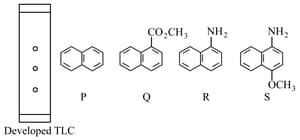
Based on the above information, the correct statement(s) is/are
Using the provided information in the following paper chromatogram:
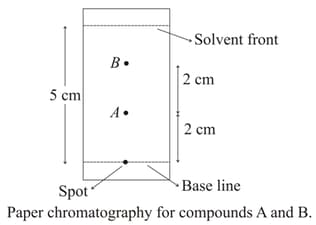
The calculated value of .
Three organic compounds and were allowed to run in thin layer chromatography using hexane and gave the following result (see figure). The value of the most polar compound is _____
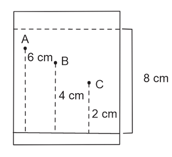
Given below are two statements:
Statement : A mixture of chloroform and aniline can be separated by simple distillation.
Statement : When separating aniline from a mixture of aniline and water by steam distillation aniline boils below its boiling point.
In the light of the above statements, choose the most appropriate answer from the options given below:
Given below are two statements: One is labelled as Assertion and the other is labelled as Reason
Assertion : Thin layer chromatography is an adsorption chromatography.
Reason : A thin layer of silica gel is spread over a glass plate of suitable size in thin layer chromatography which acts as an adsorbent. In the light of the above statements, choose the correct answer from the options given below
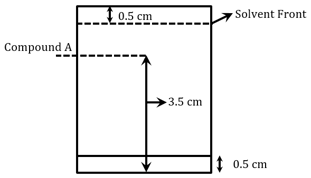
Following chromatogram was developed by adsorption of compound '' on a TLC glass plate. Retardation factor of the compound '' is _____ .
Given below are two statements:
Statement-I: Retardation factor can be measured in meter/centimetre.
Statement-II: value of a compound remains constant in all solvents.
Choose the most appropriate answer from the options given below: