MEDIUM
Earn 100
Explain the acid-base indicator constant?
Important Questions on Acids, Bases and Salts
MEDIUM
MEDIUM
HARD
MEDIUM
MEDIUM
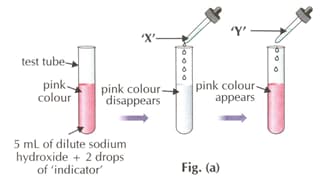
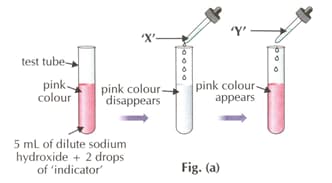
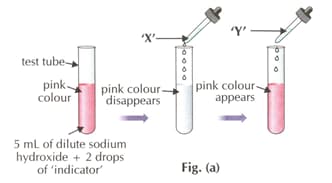
Which of the following indicators is used in the activity?
HARD
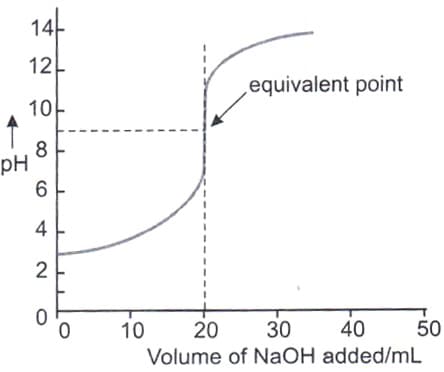
KIn value of phenolphthalein is 4.0 x 10-10. Thus, incorrect statement is
HARD
EASY
EASY
MEDIUM
HARD
Phenolphthalein
HARD
EASY
Write the quadrants in which the following points lie:
HARD
MEDIUM
EASY
An element reacts with water to form a solution which turns phenolphthalein solution pink. The element is most likely to be:
HARD