Explain the difference between complete and incomplete combustion reactions.

Important Questions on Interaction
A student performed a series of reactions between a metal and solution and recorded whether or not there was a reaction. Consider the results and answer the following question.
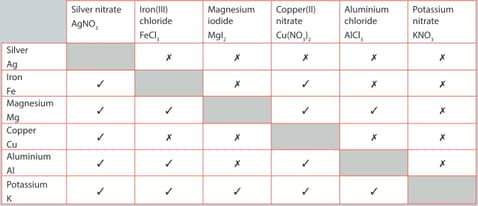
Identify the most reactive metal.
A student performed a series of reactions between a metal and solution and recorded whether or not there was a reaction. Consider the results and answer the following question.
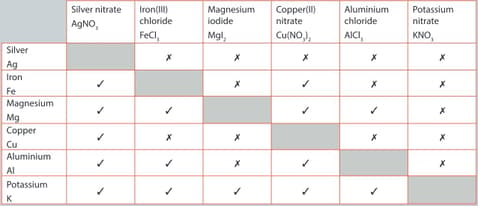
Iron is less reactive than aluminium. Provide evidence to justify this statement.
A student performed a series of reactions between a metal and solution and recorded whether or not there was a reaction. Consider the results and answer the following question.
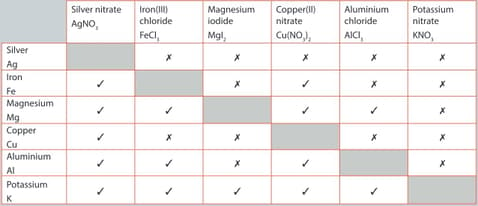
State which is the least reactive metal and give reasons for your choice.
A student performed a series of reactions between a metal and solution and recorded whether or not there was a reaction. Consider the results and answer the following question.
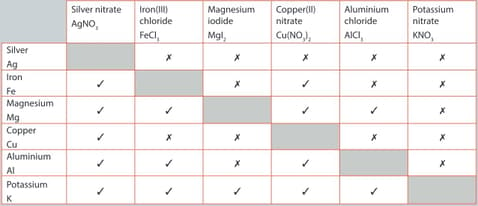
List the metals in order of most reactive to least reactive.
The single replacement reactions in given table are examples of a redox reaction.
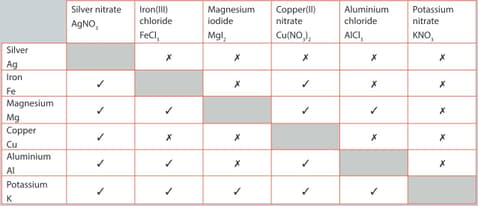
For the reaction listed below:
Silver nitrate and magnesium
Write the balanced chemical equation for the reaction. Write the two half-equations for the reaction. Identify the species being oxidized and the species being reduced.
The single replacement reactions in given table are examples of a redox reaction.
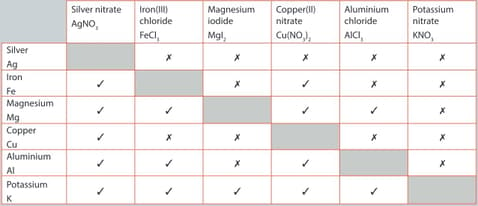
For the reaction listed below:
Aluminium and copper(II) nitrate
Write the balanced chemical equation for the reaction. Write the two half-equations for the reaction. Identify the species being oxidized and the species being reduced.
The single replacement reactions in given table are examples of a redox reaction.
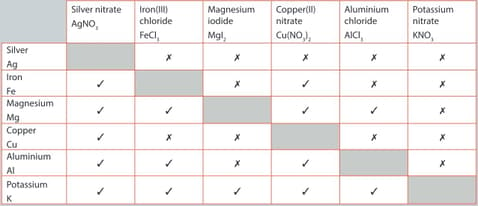
For the reaction listed below:
Potassium and magnesium iodide
Write the balanced chemical equation for the reaction. Write the two half-equations for the reaction. Identify the species being oxidized and the species being reduced.
The single replacement reactions in given table are examples of a redox reaction.
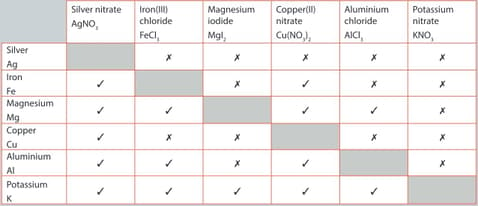
For the reaction listed below:
Iron and copper nitrate
Write the balanced chemical equation for the reaction. Write the two half-equations for the reaction. Identify the species being oxidized and the species being reduced.
