Explain the following:
Halogens have high electron affinity.

Important Questions on Periodic Table, Periodic Properties and Variations of Properties
Explain the following:
The reducing power of an element increases down in the group while decreases in a period.
Explain the following:
The size of atoms progressively becomes smaller when we move from sodium to chlorine in the third period of the Periodic Table.
Name the periodic property which relates to the:
Amount of energy required to remove an electron from an isolated gaseous atom.
Name the periodic property which relates to the:
The character of the element that loses one or more electrons when supplied with energy.
Name the periodic property which relates to the:
Tendency of an atom in a molecule to attract shared pair of electrons.
The question refers to the elements of the Periodic Table with atomic numbers to . Some of the elements are shown by letters, but the letters are not the usual symbols of the elements.

Which of these is the most electronegative element?
The question refers to the elements of the Periodic Table with atomic numbers to . Some of the elements are shown by letters, but the letters are not the usual symbols of the elements.

Which of these is a halogen?
The question refers to the elements of the Periodic Table with atomic numbers and . Some of the elements are shown by letters, but the letters are not the usual symbols of the elements.
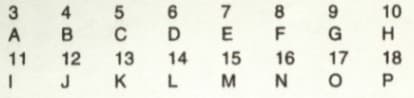
Which of these is an alkali metal?
