Explain the following term with example : Anionic detergent.
Important Questions on Chemistry
Explain the mechanism of the cleaning action of soaps.
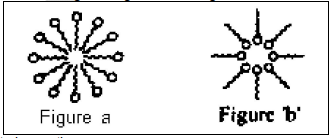
Detergents are also called surface-active agents (surfactants). These have two distinct parts: one hydrophilic spherical part and another hydrophobic long tail made of carbons chain. Two experiment ‘A’ and ‘B’ were carried out. In experiment ‘A’, surfactant was added in a beaker containing water. In experiment ‘B’, surfactant was added in a beaker containing hexane.
Following are possible results in these experiments.
I. In experiment ‘A’(see figure) ‘a’ micelle is formed, where hydrophobic part is inside the micelle and hydrophilic part is outside the micelle.
II. In experiment ‘B’(see figure ‘b’) micelle of reverse type is formed where hydrophilic part is inside the micelle and hydrophobic part is outside the micelle.
III. Micelle of reverse type is formed in experiment ‘A’.
IV. Micelles are large enough to scatter light in ‘A’
Correct observations are:
Which of the following substances cause temporary hardness in water?
Select the correct answer using the code given below.
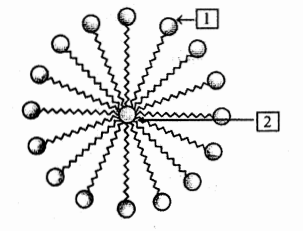
Name the structure shown in the figure. Label 1 and 2.