Explain the formation of the hydrogen molecule according to the Valence Bond Theory.
Important Questions on Chemical Bonding
The potential energy curve for formation as a function of internuclear distance of the atoms is shown below.
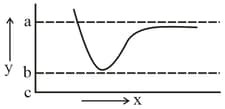
The bond energy of is :
Give a brief account of Valence Bond Theory.
Given below are two statements : One is labelled as Assertion and the other is labelled as Reason
Assertion : Zero orbital overlap is an out of phase overlap.
Reason : It results due to different orientation/ direction of approach of orbitals.
In the light of the above statements. Choose the correct answer from the options given below
What is true about molecule?
It has two lone pairs.
It has one dative bond.
It has two bonds.
It has one bond.
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
Overlapping leads to the formation of bonding molecular orbital in:

