Explain the process of sublimation by molecular model.
Important Questions on Matter
| Column A | Column B |
| (a) Molecules | (i) Water boils |
| (b) | (ii) Evaporation |
| (c) | (iii) Changes from solid to gas |
| (d) At all temperatures | (iv) Matter |
| (e) Camphor | (v) Water freezes |
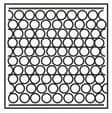
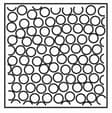
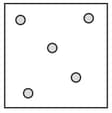
Complete the table. In the first row, draw sketches of the three states of matter in terms of the arrangement of the particles and their movement. Then describe the three states, referring to the three criteria in the first column.
| State | Solid | Liquid | Gas |
 |
 |
 |
|
| How close are particles to their neighbours? | |||
| How do the particles move? | |||
| Strength of forces between particles (strong/weak/zero) |
A small amount of smoke is blown into a small glass box. A bright light is shone into the box. When observed through a microscope, specks of light are seen to be moving around at random in the box. What evidence does this provide for the kinetic model of matter?
Which states of matter are being described here? Complete the table.
The first example has been done for you.
| Description | State or states |
| Occupies a fixed volume | Solid, liquid |
| Evaporates to become a gas | |
| Takes the shape of its container | |
| Has a fixed volume | |
| May become a liquid when its temperature changes |
Why is this model of matter sometimes described as the 'kinetic' model?
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and

