HARD
Earn 100
Explain the reaction of metals and non-metals with
(i) Acids (ii) Air (iii) Water
Important Questions on Material : Metals and Non-Metals
MEDIUM
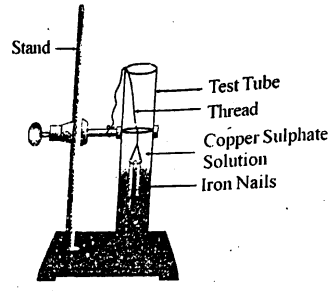
Write a chemical equation for the reaction taking place in the test tube. How does the colour of the solution change? What is the change in colour of iron nails?
MEDIUM
EASY
EASY
MEDIUM
Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas?
HARD
MEDIUM
MEDIUM
Given reason for your choice.
HARD
HARD
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
Sodium, Sulphur, Carbon, Magnesium. Which of these elements will form acidic oxides?
MEDIUM
EASY
MEDIUM
HARD
MEDIUM

