MEDIUM
Earn 100
Explain the terms isomorphism, polymorphism, anisotropy and unit cell.
Important Questions on The Solid State
MEDIUM
MEDIUM
HARD
MEDIUM
EASY
HARD
HARD
MEDIUM
(a) Aluminium crystallizes in a cubic close-packed structure. Its metallic radius is . What is the length of the side of the unit cell?
(b) Why is potassium chloride sometimes violet instead of pure white?
MEDIUM
HARD
MEDIUM
MEDIUM
(A) Crystalline solids have long range order.
(B) Crystalline solids are isotropic.
(C) Amorphous solid are sometimes called pseudo solids.
(D) Amorphous solids soften over a range of
temperatures.
(E) Amorphous solids have a definite heat of fusion. Choose the most appropriate answer from the options given below.
MEDIUM
HARD
The cubic unit cell structure of a compound containing cation and anion is shown below. When compared to the anion, the cation has smaller ionic radius. Choose the correct statement(s).
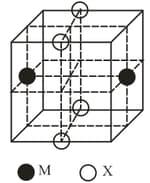
MEDIUM
MEDIUM
MEDIUM
What is the number of atoms in end centered unit cell?
MEDIUM
MEDIUM
Given below are two statements. One is labelled as
Assertion and the other is labelled as Reason .
Assertion Sharp glass edge becomes smooth on heating it up to its melting point.
Reason The viscosity of glass decreases on melting.
Choose the most appropriate answer from the options given below.
EASY

