Explain what is meant by the wave-particle duality of electromagnetic radiation.

Important Questions on Quantum Physics
Explain how the photoelectric effect gives evidence for the wave-particle duality of electromagnetic radiation.
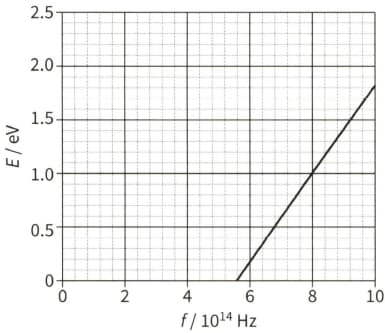
State what is meant by the de Broglie wavelength of an electron.
The diagram shows the principles of an electron tube used to demonstrate electron diffraction.
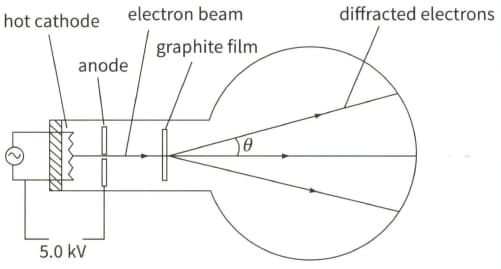
Calculate the kinetic energy $E$ (in joules) of the electrons incident on the graphite film.
The diagram shows the principles of an electron tube used to demonstrate electron diffraction.
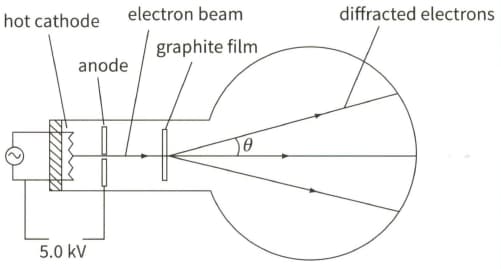
Show that the momentum of an electron is equal to $\sqrt{2 E m_{e}}$ where $m_{\mathrm{e}}$ is the mass of an electron, and hence calculate the momentum of an electron. $\left(m_{\mathrm{e}}=9.11 \times 10^{-31} \mathrm{~kg}\right)$
The diagram shows the principles of an electron tube used to demonstrate electron diffraction.
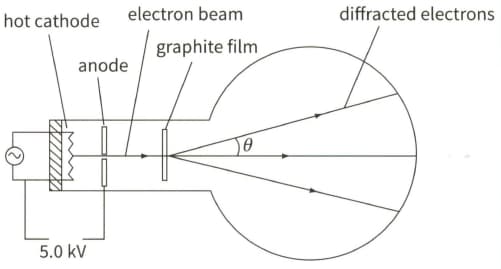
Calculate the de Broglie wavelength of the electrons.
