HARD
Diploma
IMPORTANT
Earn 100
Explain why the dark lines of an absorption spectrum have the same wavelengths as the bright lines of an emission spectrum for the same element

Important Questions on Atomic, Nuclear and Particle Physics
HARD
Diploma
IMPORTANT
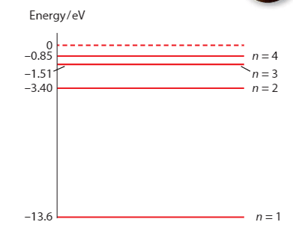
Calculate the wavelength of the photon emitted in a transition from . Use the given figure
HARD
Diploma
IMPORTANT
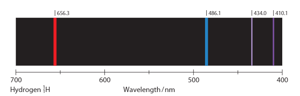
Refer to the figure, explain why the distance between the emission lines of hydrogen decreases as we move to the right
MEDIUM
Diploma
IMPORTANT
A hydrogen atom is in its ground state. Explain the term 'ground state'.
HARD
Diploma
IMPORTANT
A hydrogen atom is in its ground state. Explain the term 'ground state'. Photons of energy are directed at hydrogen gas in its ground state. Suggest what, if anything, will happen to the hydrogen atoms.
HARD
Diploma
IMPORTANT
A hydrogen atom is in its ground state. Explain the term 'ground state'.
In another experiment, a beam of electrons of energy is directed at hydrogen gas atoms in their ground state. Suggest what, if anything, will happen to the hydrogen atoms and the electrons in the beam.
MEDIUM
Diploma
IMPORTANT
State the electric charge of the nucleus
MEDIUM
Diploma
IMPORTANT
State what is meant by the term "isotope'?
MEDIUM
Diploma
IMPORTANT
State two ways in which the nuclei of isotopes differs from each other( other than they have different neutrons.
