EASY
JEE Advanced
IMPORTANT
Earn 100
Figure shows the energy levels and of an atom where, is the ground state. A red line in the emission spectrum of the atom can be obtained by an energy level change from to . A blue line can be obtained by which of the following energy level change?
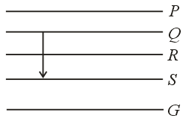

(a) to
(b) to
(c) to
(d) to
50% studentsanswered this correctly

Important Questions on Modern Physics
EASY
JEE Advanced
IMPORTANT
An electron makes a transition from orbit to the orbit of a hydrogen atom. The wave number of the emitted radiations ( Rydberg's constant ) will be
HARD
JEE Advanced
IMPORTANT
The wavelength of radiation emitted is when an electron jumps from the third to the second orbit of a hydrogen atom. For the electron jump from the fourth to the second orbit, the wavelength of radiation emitted will be,
MEDIUM
JEE Advanced
IMPORTANT
When an electron in a hydrogen atom is excited from its fourth to fifth stationary orbit, the change in angular momentum of electron is (take, Planck's constant, ),
EASY
JEE Advanced
IMPORTANT
The concept of stationary orbits was proposed by,
EASY
JEE Advanced
IMPORTANT
The ratio of the speed of the electrons in the ground state of hydrogen to the speed of light in vacuum is,
MEDIUM
JEE Advanced
IMPORTANT
The ionisation potential of -atom is . When it is excited from ground state by monochromatic radiations of , the number of emission lines will be (according to Bohr's theory),
MEDIUM
JEE Advanced
IMPORTANT
The excitation energy of a hydrogen like ion in its first excitation state is . The energy needed to remove the electron from the ion in ground state is,
EASY
JEE Advanced
IMPORTANT
In Bohr's model, the atomic radius of the first orbit is . Then, the radius of the third orbit is,
