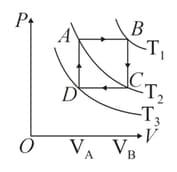
Figure shows three isotherms at temperature . When one mole of an ideal monatomic gas is taken through the paths and , find the change in internal energy , the work done by the gas and the heat absorbed by the gas in each path. Also, find these quantities for complete cycle .


Important Questions on Thermodynamics
When a thermodynamic system is taken from an initial state to a final state along the path , as shown in the given figure, the heat energy absorbed by the system is and the work done by the system is . If the same system is taken along the path , the value of .
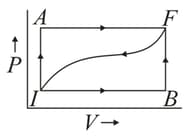
Find the work done along the path .
When a thermodynamic system is taken from an initial state to a final state along the path , as shown in the given figure, the heat energy absorbed by the system is and the work done by the system is . If the same system is taken along the path , the value of .
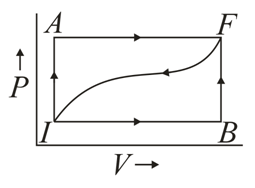
If for the curved path , how much heat energy is lost by the system along this path?
When a thermodynamic system is taken from an initial state to a final state along the path , as shown in the given figure, the heat energy absorbed by the system is and the work done by the system is . If the same system is taken along the path , the value of .
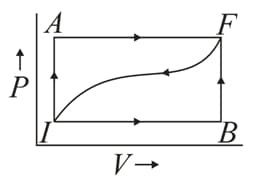
If , what is
When a thermodynamic system is taken from an initial state to a final state along the path , as shown in the given figure, the heat energy absorbed by the system is and the work done by the system is . If the same system is taken along the path , the value of .
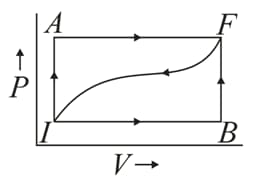
If , what is for the process and
A gas is undergoing an adiabatic process. At a certain stage , the values of volume and temperature are and , respectively. From the details given in the graph, find the value of and .

In a cylinder, moles of an ideal monatomic gas initially at and expands until its volume doubles. Compute the work done if the expansion is isothermal
In a cylinder, moles of an ideal monatomic gas initially at and expands until its volume doubles. Compute the work done if the expansion is adiabatic?
