MEDIUM
Earn 100
Fill in the blank by using the correct words/terms given in the brackets.
A water-soluble base produces
ions in solution. (Hydrogen/Hydroxyl)
50% studentsanswered this correctly
Important Questions on Acids, Bases and Salts
EASY
MEDIUM
EASY
MEDIUM
Four solutions A, B, C, and D have pH 2, 6. 7, and 13 respectively:
Which solution will have the highest number of hydronium ions?
EASY
EASY
MEDIUM
What is an alkali? Do basic solutions also have (aq) ions? If yes, then why are these basic?
EASY
Which of the following is a strong acid?
MEDIUM
MEDIUM
MEDIUM
The bulb is not glowing in this experimental setup. Because:
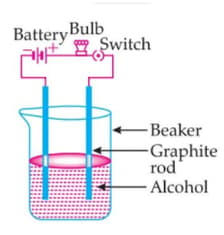
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
HARD

