HARD
JEE Main
IMPORTANT
Earn 100
Find the adiabatic exponent , for a mixture consisting of moles of a monatomic gas and moles of a gas of rigid diatomic molecules.

Important Questions on THERMODYNAMICS AND MOLECULAR PHYSICS
HARD
JEE Main
IMPORTANT
A thermally insulated vessel, with gaseous nitrogen, at a temperature , moves with velocity . How much (in per cent) and in what way will the gas pressure change, on a sudden stoppage of the vessel?
HARD
JEE Main
IMPORTANT
Calculate, at the temperature, ,
the root mean square velocity and the mean kinetic energy of an oxygen molecule, in the process of translational motion,
the root mean square velocity of a water droplet, of diameter , suspended in the air.
HARD
JEE Main
IMPORTANT
A gas, consisting of rigid diatomic molecules, is expanded adiabatically. How many times has the gas to be expanded, to reduce the root mean square velocity of the molecules, times?
HARD
JEE Main
IMPORTANT
The mass , of nitrogen, is enclosed in a vessel, at a temperature . What amount of heat has to be transferred to the gas, to increase the root mean square velocity of its molecules, times?
HARD
JEE Main
IMPORTANT
The temperature of a gas, consisting of rigid diatomic molecules, is . Calculate the angular root mean square velocity of a rotating molecule, if its moment of inertia is equal to .
HARD
JEE Main
IMPORTANT
A gas consisting of rigid diatomic molecules, was initially under standard conditions. Then the gas was compressed adiabatically times. Find the mean kinetic energy of a rotating molecule in the final state.
HARD
JEE Main
IMPORTANT
An ideal gas goes through a cycle consisting of alternate isothermal and adiabatic curves. The isothermal processes proceed at temperatures and Find the efficiency of such a cycle, if in each isothermal expansion, the gas volume increases in the same proportion.
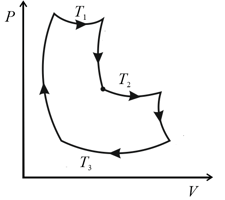
HARD
JEE Main
IMPORTANT
Find the efficiency of a cycle, consisting of two isochoric and two adiabatic lines, if the volume of the ideal gas changes, times, within the cycle. The working substance is nitrogen.
