Find the equivalent weight of in each of the following reactions:
(a) or
(b)
(c)
Equation (c) is the sum of equations (a) and (b). What is the relationship between the answer to (c) and the answers to (a) and (b)?

Important Questions on Miscellaneous Problems for Revision
Find the equivalent weight of in the reaction:
What mass of is oxidised by of ?
The time required for completion of a first-order reaction at is equal to that required for its completion at . If the pre-exponential factor for the reaction is , calculate its rate constant at and also the energy of activation.
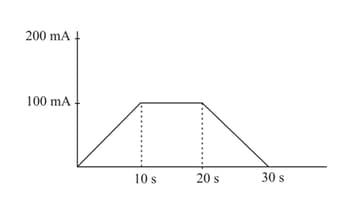
In a -voltameter, the mass deposited in seconds is grams. If the time-current graph is as shown in the figure, calculate the electrochemical equivalent of .
of at and pressure dissolves in of water at the same temperature, when the pressure of is . Calculate the molal concentration of in a solution over which the partial pressure of is Report your answer after multiplying by .
The voltage of the cell, If , report correct up to one place of decimal.
and .
