HARD
Earn 100
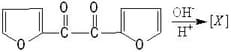
Find the last product [D] in reaction sequence
(a)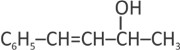
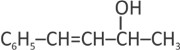
(b)

(c)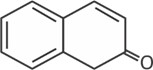

(d)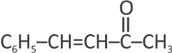
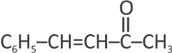
50% studentsanswered this correctly
Important Questions on Aldehydes, Ketones and Carboxylic Acids
HARD
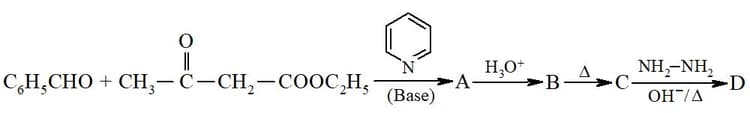
Consider the reaction sequence below:
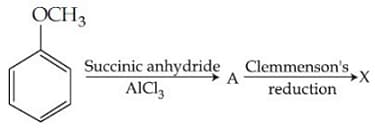
X is :
MEDIUM
The major product of the following reaction is:
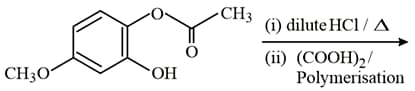
EASY
Aldehydes or ketones when treated with the product formed is
HARD
Consider the reactions:
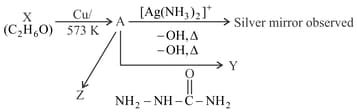
Identify and .
HARD
Explain the mechanism of the Claisen Schmidt reaction.
MEDIUM
Which of the following reagents would distinguish cis-cyclopenta-1, 2-diol from the trans-isomer?
MEDIUM
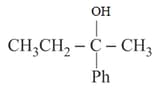
cannot be prepared by:
MEDIUM
Which is the main organic product obtained by the reaction of trichloro ethanal with calcium hydroxide?
MEDIUM
Reaction of benzaldehyde with acetic anhydride in the presence of base is known as _____ and the product
EASY
Mention the reagents used for the following conversions:
(m)
(n)
(o)
(p)
(q)
(r)
EASY
Write chemical equations to illustrate the following name reactions: Benzoin condensation.
EASY
Which compound does not give Benedict test?
EASY
Benedict's solution is not reduced by
HARD
A keto ester with molecular formula on treatment with does not give iodoform but on boiling with dilute KOH gives a compound with molecular formula which upon acidification followed by heating undergoes decarboxylation to give acetone. The keto ester is
HARD
Claisen condensation is not given by
MEDIUM
Which of the following reactions can be used to change benzaldehyde and acetic anhydride to cinnamic acid?
MEDIUM
In the context of the rearrangement of an oxime of a ketone to an amide (represented below)
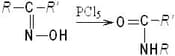
Which of the following statement is/are correct?
HARD
Conversion of benzaldehyde to 3-phenylprop-2-en-1-oic acid is