MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
Find the lifting power of a balloon filled with helium at and . (Density of air ).

Important Questions on States of Matter: Gases and Liquids
HARD
JEE Main/Advance
IMPORTANT
The drain cleaner, Drainex contains small bits of aluminium which react with caustic soda to produce dihydrogen. What volume of dihydrogen at and one bar will be released when of aluminium reacts?
MEDIUM
JEE Main/Advance
IMPORTANT
What will be the pressure of the gas mixture when of at and of oxygen at are introduced in a vessel at ?
MEDIUM
JEE Main/Advance
IMPORTANT
Density of a gas is found to be at and pressure. What will be its density at STP?
MEDIUM
JEE Main/Advance
IMPORTANT
A mixture of dihydrogen and dioxygen at one bar pressure contains by weight of dihydrogen. Calculate the partial pressure of dihydrogen.
MEDIUM
JEE Main/Advance
IMPORTANT
A vessel contains and in the molar ratio of respectively. This mixture of gases is allowed to diffuse through a hole, find composition of the mixture coming out of the hole.
MEDIUM
JEE Main/Advance
IMPORTANT
In a tube of length having identical holes at the opposite ends. and are made to effuse into the tube from opposite ends under identical conditions. Find the point where gases will meet for the first time.
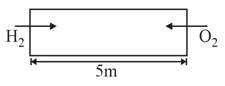
MEDIUM
JEE Main/Advance
IMPORTANT
Pressure of of an ideal gas A at is found to be bar. When of another ideal gas B is introduced in the same flask at same temperature the pressure becomes . Find a relationship between their molecular masses.
HARD
JEE Main/Advance
IMPORTANT
of a gas at occupied the same volume as of dihydrogen at at the same pressure. What is the molar mass of the gas?
