MEDIUM
Earn 100
Find the number of molecules or ions in which d orbitals is/are not used in hybridisation
50% studentsanswered this correctly
Important Questions on Chemical Bonding
EASY
EASY
EASY
EASY
EASY
MEDIUM
MEDIUM
EASY
EASY
EASY
EASY
MEDIUM
EASY
The hybridisations of and shown in the following compound
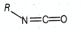
respectively, are
MEDIUM
EASY
EASY
EASY
In the following molecules,
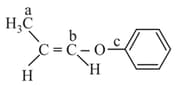
Hybridisation of carbon and respectively are :
EASY
MEDIUM
EASY

